Professor Barbara Zieger, MD, MME (Master of Medical Education)
Head, Section for Hemostaseology
E: barbara.zieger@uniklinik-freiburg.de
T: +49 761 270 43000
F: +49 761 270 45820
Medical Center – University of Freiburg
Center for Pediatrics
Division of Pediatric Hematology and Oncology
Section for Hemostaseology
Mathildenstr.1
79106 Freiburg
Germany
Regulation of hemostasis is a key element of a healthy life. Inherited or acquired disorders of hemostasis can lead to increased bleeding and prolonged wound healing. Our research focuses on biochemical and molecular genetical characterization of inherited platelet disorders (IPDs), inherited von Willebrand disease and acquired von Willebrand syndrome to assess patients’ risk profiles and to optimize therapy. Additionally, we have identified several novel disease-causing variants in various inherited platelet disorders. Our work also comprises basic research on murine and human septins (GTP-binding proteins) which are part of the cellular cytoskeleton and which seem to be involved in secretion and membrane processes. Our aim is to understand the impact of septins in platelet function and human disease.
TEAM

- Doris Böckelmann (PhD)
- Katharina Neubauer (PhD)
- Anja Kahle (Technican)
- Eileen Lerner (B.Sc., Technican)
- Antonia Lenz (MD student)
- Salome Fels (MD student)
- Felix Sobotta (MD student)
- Mira Wolters (MD student)
Former lab members
- Kirstin Sandrock-Lang (PhD)
- Ingrid Bartsch (PhD)
- Susanne Bläser (PhD)
RESEARCH THEMES
In many patients who present with severe bleeding symptoms, the cause remains unclarified for many years and the patients suffer significantly from the symptoms and the associated complications (re-operation, inflammation, wound healing disorders). We have established innovative methods to investigate primary hemostasis disorders, including aggregometry, flow cytometry of platelets, vWF multimeric analyses and high-throughput sequencing. For molecular genetic analysis, we have established a panel-based next generation sequencing (NGS) approach, comprising 95 genes associated with IPDs and von Willebrand disease. Included in the panel are genes coding for human septins which assemble into cytoskeletal filaments and are involved in multiple processes (i.e. platelet and membrane processes).
CURRENT PROJECTS
Biochemical and molecular genetic characterization (NGS) of patients with inherited defects of the primary hemostasis (platelet disorders and von Willebrand disease)
Inherited platelet disorders (IPDs) comprise a highly heterogeneous group of disorders affecting platelet number and function. IPDs are phenotypically and biochemically highly diverse. The extent of bleeding symptoms (hematoma, gastrointestinal bleeding, menorrhagia, intracranial bleeding) can vary widely. After trauma or surgical intervention, life-threatening bleeding can occur leading to reoperations or even resuscitation. IPDs may be syndromic or associated with risk to develop malignant disorders. The precise clinical and biochemical characterization and identification of the molecular genetic defect of the inherited platelet disorder are important for stratifying the patients’ risk profile and for personalized therapeutic options. Many patients also suffer from von Willebrand disease and extensive bleeding complications. Therefore, a comprehensive phenotypical and molecular genetic characterization of these patients is elementary to provide adequate therapy. To identify the molecular genetic defect of primary hemostasis defects we use next generation sequencing (gene panel and whole exome).
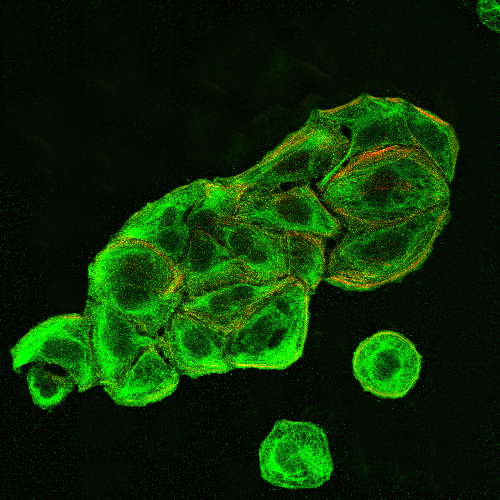
Phenotypical and biochemical platelet characterization of a patient with Glanzmann thrombasthenia (Cesari et al., J Blood Transfusions Dis. 2019; 2(1): 36-39).
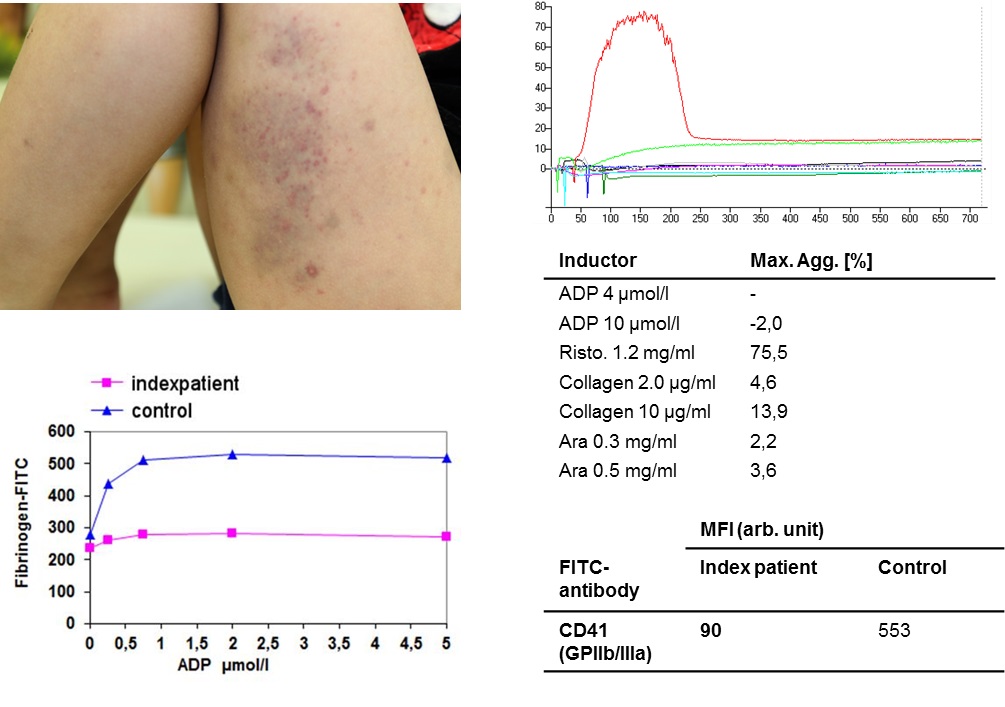
Acquired von Willebrand syndrome and thrombocytopathy in patients with Ventricular Assist Device (VAD), Extracorporeal Life Support (ECLS) or Extracorporeal Membrane Oxygenation (ECMO)
Severe bleeding symptoms are one of the main complications in patients with VAD, ECLS or ECMO. Our working group identified for the first time Acquired von Willebrand syndrome (AvWS) as the main cause of these bleeding symptoms and published these results for large cohorts with VAD, ECLS or ECMO. In addition, we identified thrombocytopathy (platelet secretion defect) in these patients. AvWS and thrombocytopathy seem to be due to the increased shear stress caused by these devices leading to hemolysis and activation of primary and secondary hemostasis. Identifying these acquired disorders leads to improved diagnostic and therapeutical algorithms and better clinical outcome. In addition, these results may help to develop devices which will lead to less shear stress.
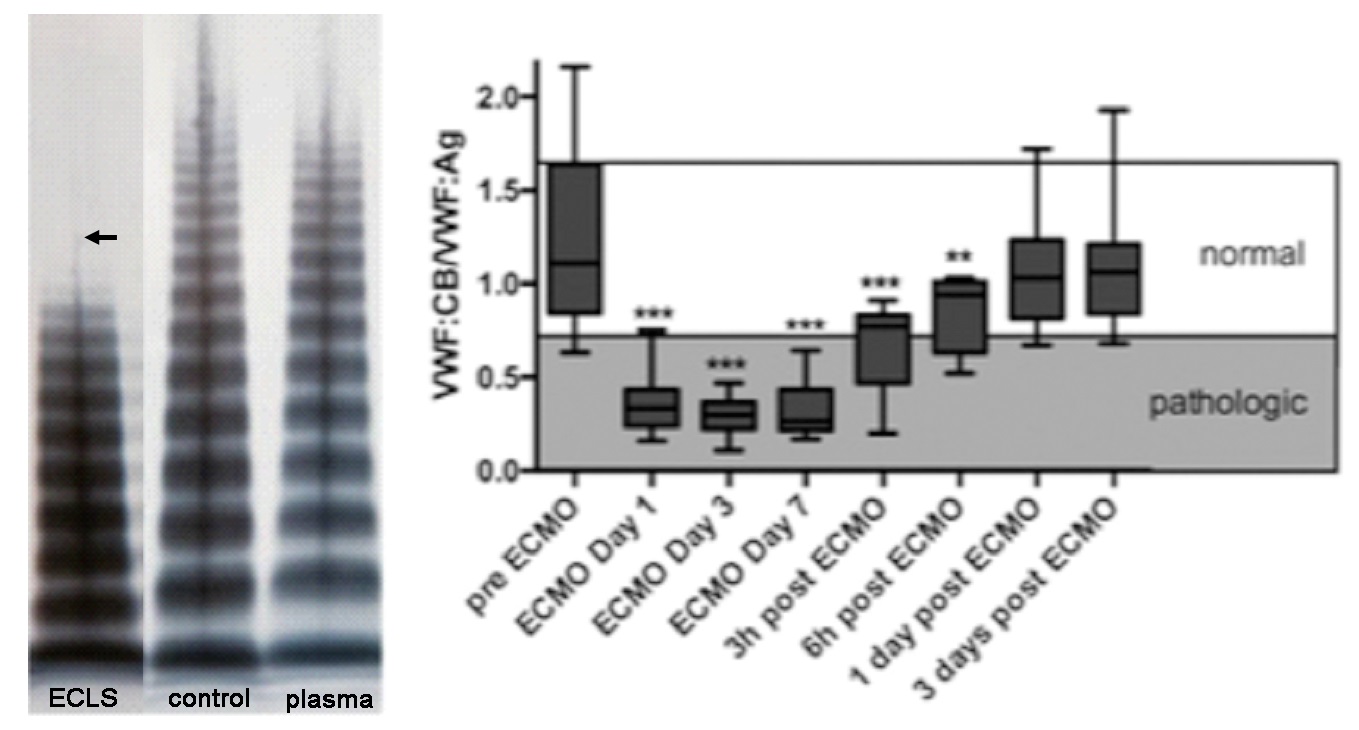
Von Willebrand factor (vWF) multimeric analysis (ECLS: loss of high molecular weight multimers) and ratio of vWF:collagen binding activity/vWF:antigen pre, during and post ECMO (Heilmann et al., Intensive care medicine. 2012; 38(1):62-8; Kalbhenn et al., J Heart Lung Transplant. 2018; 37(8):985-91.
Septins
Septins are a family of filamentous GTPases with 13 members in mammals. These proteins form oligomers and complexes with other proteins to serve as multi-molecular scaffolds and recruit components of signaling pathways. In platelets, septins are highly expressed and are possibly involved in vesicle trafficking. Dysregulation of septins leads to a variety of human diseases and amongst others to bleeding disorders. Originally, our group cloned 4 of these septins (SEPT4, SEPT5, SEPT8, SEPT11) and investigated septin expression and septin interaction with other proteins. We cloned SEPT5 as one of the first human septins and described the expression of other septins in human endothelial cells and platelets. In particular, we showed that some septins (SEPT4, 5, and 8) are localized around the platelet α-granules and move to the platelet surface after activation, suggesting a role of for these septins in platelet function. In this context we identified the first patient with a SEPT5-defect suffering from life-threatening bleedings due to co-deletion of SEPT5 and GPIbβ (encoding for the subunit of the platelet von Willebrand factor receptor GPIb/IX). This boy showed a platelet secretion defect and developmental retardation. To identify the impact of septins in bleeding disorders and to contribute to a better understanding of septin physiology, we perform extensive platelet function analyses in septin-knockout mouse models.
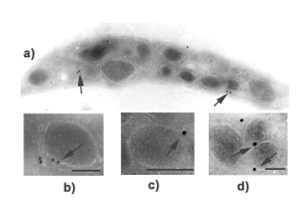
Transmission electron microscopy and immunogold labeling of SEPT4 (arrows) in human platelets (Blaser et al., Thromb Haemost. 2004; 91(5):959-66).
COLLABORATIONS, CO-OPERATIONS AND NETWORKS
- Johan Heemskerk (PhD), CARIM, Maastricht, Netherlands
- Albert Sickmann (PhD), isas, Dortmund, Germany
- Kerstin Jurk (PhD), Mainz, Germany
- Axel Schlagenhauf (PhD), Medizinische Universität Graz, Graz, Austria
- Willem Ouwehand (PhD), Cambridge, United Kingdom
SELECTED RECENT PUBLICATIONS
- Neubauer K, Jurk K, Petermann V, Kumm E, Zieger B. Impaired platelet function in Sept8-deficient mice in vitro. Thrombosis and Haemostasis. 2020; in press
- Bockelmann D, Naz A, Siddiqi MYJ, Lerner E, Sandrock-Lang K, Shamsi TS, Zieger B. Bernard-Soulier syndrome in Pakistan: Biochemical and molecular analyses leading to identification of a novel mutation in GP1BA. Haemophilia. 2018;24(1):e18-e22.
- Geisen U, Brehm K, Trummer G, Berchtold-Herz M, Heilmann C, Beyersdorf F, Schelling J, Schlagenhauf A, Zieger B. Platelet Secretion Defects and Acquired von Willebrand Syndrome in Patients With Ventricular Assist Devices. J Am Heart Assoc. 2018;7(2).
- Kalbhenn J, Schlagenhauf A, Rosenfelder S, Schmutz A, Zieger B. Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: Rapid onset and fast recovery. The Journal of Heart and Lung Transplantation. 2018;37(8):985-91.
- Bender M, Stritt S, Nurden P, van Eeuwijk JM, Zieger B, Kentouche K, Schulze H, Morbach H, Stegner D, Heinze KG, Dutting S, Gupta S, Witke W, Falet H, Fischer A, Hartwig JH, Nieswandt B. Megakaryocyte-specific Profilin1-deficiency alters microtubule stability and causes a Wiskott-Aldrich syndrome-like platelet defect. Nature Communications. 2014;5:4746.
- Bartsch I, Sandrock K, Lanza F, Nurden P, Hainmann I, Pavlova A, Greinacher A, Tacke U, Barth M, Busse A, Oldenburg J, Bommer M, Strahm B, Superti-Furga A, Zieger B. Deletion of human GP1BB and SEPT5 is associated with Bernard-Soulier syndrome, platelet secretion defect, polymicrogyria, and developmental delay. Thrombosis and Haemostasis. 2011;106(3):475-83.
- Geisen U, Heilmann C, Beyersdorf F, Benk C, Berchtold-Herz M, Schlensak C, Budde U, Zieger B. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. European Journal of Cardio-Thoracic Surgery.2008;33(4):679-84.
A complete list of publications is available here: https://pubmed.ncbi.nlm.nih.gov/?term=zieger+b
FUNDING
DFG (ZI 486/4-1 and ZI 486/8-1)
Society of Thrombosis and Haemostasis Research– Erwin Deutsch Award